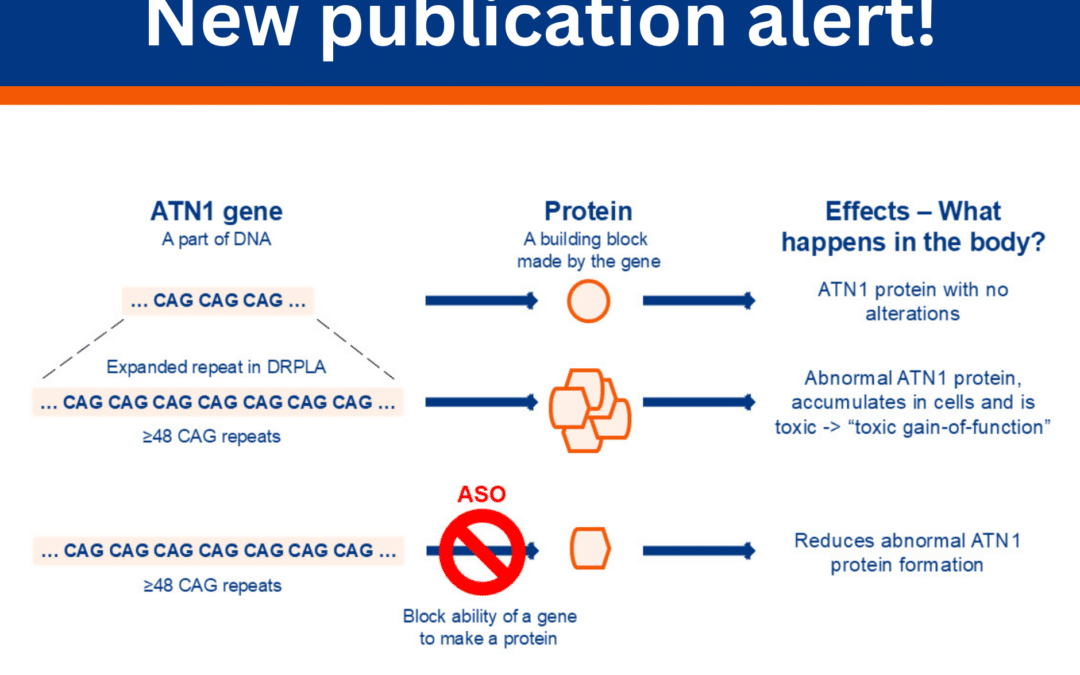
The most comprehensive description of symptoms and findings from clinical tests, brain scans, and blood tests from people living with DRPLA has been published by Dr Shi-Rui Gan and his collaborators based in China. They enrolled 116 people living with DRPLA across...